药厂车间的制药用水和蒸汽系统设计是保证车间产品质量的关键,也是顺利通过GMP认证的基本要求。因此,在设计时也要充分考虑风险。德斯特给大家分享下最新的制药车间制药用水和蒸汽系统设计案例,仅供大家参考。
Pharmaceutical water is the most widely used ingredient in drug manufacturing and the main component in equipment/system cleaning.Therefore, systems for the production of pharmaceutical water constitute a key component in every manufacturing facility. The nature of producing pharmaceutical waters is to minimize or eliminate potential sources of contamination. This Guide considers this and the means by which engineers can design out, or ensure control of the risk.
制药用水在药品生产中是使用最广泛的成份,并且在设备/系统清洗中也是主要组份。因此,制药用水的生产系统在所有制药企业中构成一个关键组份。生产制药用水的本质是将潜在的污染源降到最低或者消除。本指南认为其以及工程师使用的工具可以设计或者确保控制风险。
The quality of Pharmaceutical Water and Steam is not only critical from a regulatory point of view, but also from a financial point of view. The Pharmaceutical Water and Steam specification has the largest impact on lifecycle costs of the system.
制药用水和蒸汽的质量不仅是调控观点的关键,在经济观点上也是如此。制药用水和蒸汽技术指标对系统整个过程的成本有巨大影响。
It must be demonstrated that all pharmaceutical waters (non-compendial and United States Pharmacopoeia(USP)monograph compendial waters) can be produced consistently to specification. Establishing the level of microbial control needed in a pharmaceutical water and steam system used in the manufacture of a nonsterile product requires an understanding of both the use of the product and the manufacturing process.
必须证明可以在相同技术指标下始终如一地生产所有制药用水(非药典和 USP 药典各论用水)。制造非无菌产品时所用的制药用水或蒸汽系统中,建立必须的微生物防治水平,对产 品用途和生产工艺都需要了解。
Manufacturers need to define the appropriate water purity based upon sound process understanding and system equipment capability. They must determine the specific purification capability for each processing step, the limitations of the unit operation, and the critical parameters, which affect the specified water/steam quality - chemically, physically, or biologically. Expert QA advice should be sought to provide further details about this important area.
制造商需要依据合理的工艺理解和系统设备性能来确定适当的水质纯度。他们必须确定那些可以影响特定水/蒸汽(化学的,物理学的或生物学的)质量的每个工艺步骤特定的纯化性 能、设备操作的限制和关键参数。应该向 QA 专家征求关于该重要区域更进一步细节的建议。
USP covers two compendial water qualities (USP Purified Water and USP Water for Injection). This Guide supports both these water qualities plus additional non-compendial waters including "Drinking Water". It is common practice to name non-compendial waters (exclusive of "Drinking Water") used in pharmaceutical manufacturing by the final treatment step (i.e.Reverse Osmosis/RO water, deionized water/DI water, etc.).
USP 包含两种药典规定用水药典规定用水质量(USP纯水和 USP注射用水)。本指南即支持 这两种水质,另外附加包括“饮用水”在内的非药典规定用水药典规定用水。 通常习惯骨 终处理步骤(即,反渗透水/RO 水,去离子水/ DI 水,等等)来命名在制药生产中使用的非 药典规定用水药典规定用水(“饮用水”除外)。
Guidance on establishing specifications for monographed USP water is provided in the United States Pharmacopoeia (USP). Additionally, the FDA Guide to Inspections of High Purity Water Systems (which was developed for FDA personnel) also provides useful information to the user.
关于制定 USP 各论用水的技术指标的指南在 USP 中提供。此外,FDA 高纯度水系统的检查指南(由 FDA 工作人员制定)也为用户提供有用信息。
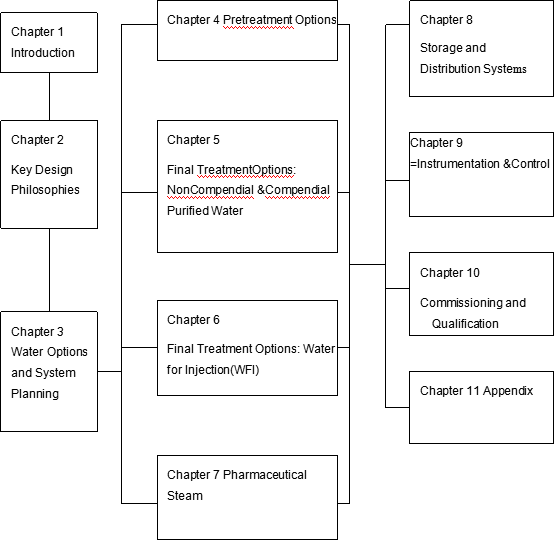
Figure 1-1 Pharmaceutical Water and Steam Baseline Guide Structure
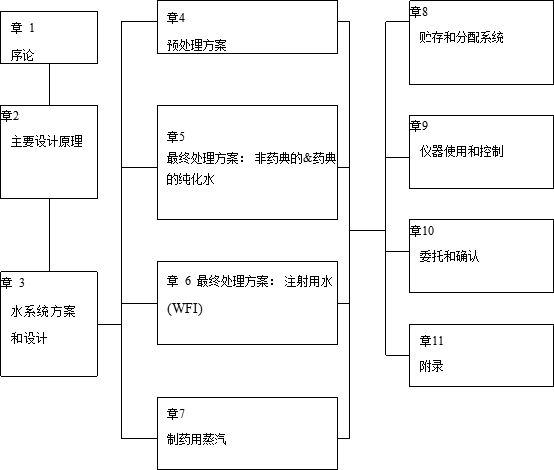
表 1-1 制药用水和蒸汽系统指南